Imagine a laboratory with liters of reagents and samples, where each operation takes place in individual vessels and instruments. What if all of the laboratory functions could be integrated in a single small portable circuit, or chip, with higher efficiencies, extremely low material consumption (down to nano/pico-liters in fluid volume), less waste production, much faster response time, and more control over the operating condition with real-time monitoring capabilities?
Lab-on-a-chip (LOC) devices or Micro Total Analysis Systems (μTAS) are a subcategory of Microelectromechanical systems (MEMS) devices. LOCs may use nano/microfluidics, the technology of fluid manipulation in microscopic scale channels with dimensions of tens of nano to micrometers. To get some idea on the scale comparison, a human hair is about 100 micron thick.
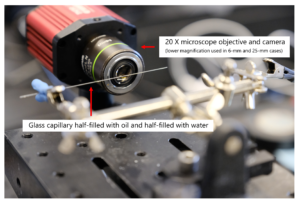
Microfluidics provides high-resolution optical access to fluid dynamics with an unprecedented level of control over the operating conditions (temperature, pressure, concentration, pH, etc.) as well as automation, and high throughput analysis with parallelization capabilities. According to the scaling laws, surface-to-volume ratio increases at small scales leading to accelerated heat and molecular transport. While the dimensions of the individual channels are small, a micro process engineering device (microstructured reactor) can contain many thousands of such channels with the overall length on the scale of meters.
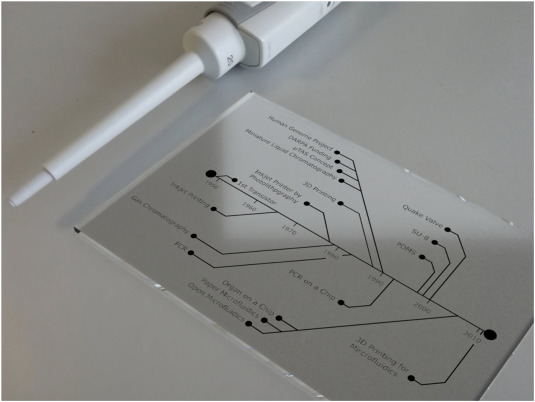
Naturally-optimized microfluidics can be found in nature – such as in blood vascular networks, natural leaves that contain complex venation networks, spider web formations, and fractured rocks and reservoirs. Microfluidics is also applied to everyday objects – such as inkjet printers, e-readers, and point-of-care diagnostic devices such as home pregnancy test kits, blood glucose monitoring strips, DNA, RNA analysis, and protein characterization systems.
This technology is developing very quickly for point-of-care diagnosis (e.g. HIV infection diagnosis), sensing and analysis (e.g. volatile organic compounds (VOC)) applications, in vitro study of drugs and pharmaceutical compounds by simulating body conditions (organ-on-chip). Microfluidics is also applied for synthesis of smart materials (e.g. nanoparticles; solid, porous and non- spheres; hierarchical composites; hydrogels) with precise control over the size, shape, structure, and porosity over conventional methods. The same approach is applied for 3D bioprinting of biomaterials towards replicating organs and tissues.
In the case of chemical reactions and operating conditions (temperature, pressure, etc,) that are inaccessible at larger volumes due to safety concerns, microfluidics with an intrinsic safety of microstructured reactors is a ‘smart’ alternative. For instance, the detonation of the stoichiometric mixture of two volume units of hydrogen gas and one volume unit of oxygen gas does not propagate in microchannels with a sufficiently small diameter.
At Interface Fluidics, we are working at the intersection of nano and microfluidics. With our team of interdisciplinary scientists and engineers, our technology has been adapted for high-temperature high-pressure fluid analysis and imaging with applications for the oil and gas industry. Interface Fluidics employs proprietary nanotechnology platforms to economically screen the fluid behavior and optimize process parameters at the nano-scale. This helps to inform operators on downhole fluid decisions, using Interface’s rapid and accurate testing capabilities. The same technology can be deployed to other sectors such as chemical and pharmaceutical producers to help them with their fluid and material selection decisions by precise analysis through online monitoring of fluid behaviors and fluid-fluid /fluid-surface interactions at the molecular level.
Are you interested in learning how Interface’s technology could better equip you for making strategic decisions before going to the field? Connect with us today to have a technical discovery meeting.
Written by
Zahra Barikbin
I’m a senior scientist with Interface. My background is in chemical engineering with extensive experience in developing and deployment of innovative technologies. I help lead all phases of R&D projects (design of experiments and processes, operations and optimization with simulation activities).